Since early 2018, Suggestic has been investing in biological age measurement and understanding precision lifestyle factors to slow, or even reverse, the rate of aging. These investments will start to have a public face this year and are then likely to imbue all of our products and services. I’m bullish about the potential and size of the “delaying death and aging”/”preserving youth and vitality” market. The science and technology is moving exponentially.
Suggestic has always been focused on bringing Hippocrates “Let your food be your medicine and let your medicine be your food.” into the digital networked era.
However we concluded many years ago that 1) biological age is a fantastic endpoint to determine what is truly “healthy”, and that 2) we could move the biological age needle, starting with individualized food choices.
Over the coming two months I’ll be introducing the groundwork to understand our vision. A reasonable place to begin is the ‘9 Hallmarks of Aging’.
Even with the exponential rate in the scientific understanding of aging, it’s still mysterious. There’s no single theory of aging. Instead, there are hundreds. However, there has been enough of a consensus on the “hallmarks” of aging to foster discussion and to be targeted for scientific discovery.
This article will briefly explain each of the hallmarks.
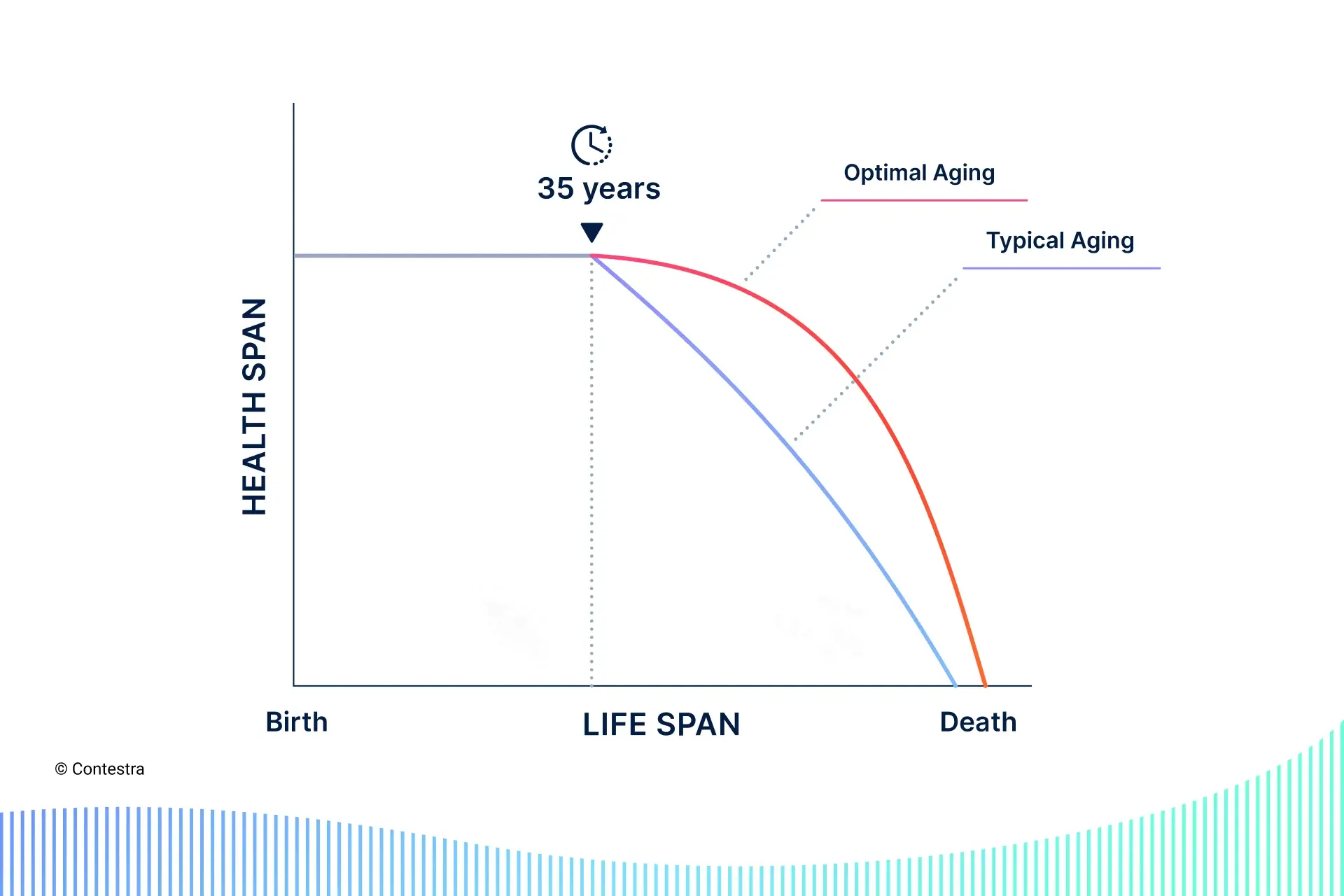
Before explaining each hallmark though, I’ll introduce the concept of ‘biological age’, and the two leading schools of thought on what aging is.
There are two systems of counting age:
- chronological age — the number of times the Earth has went around the Sun since we were born. This is the number found in our passports.
- biological age — our body’s “time-dependent functional decline”, relative to the average person in a population matching our chronological age (e.g. we could have a chronological age of 40 but a biological age of 32, which would be good because we’d have the same all-cause mortality and morbidity risk as the average person of chronological age 32, not 40)
As previously mentioned, most aging theories fall into two schools of thought. It’s likely that aging is an overlap of both schools.
- programmed theory — the idea that living too long leads to evolutionary disadvantages, and therefore aging and death are genetically programmed to occur
- damage theory — the idea that the accumulation of damage in cells and organs, caused by environment, leads to aging and death
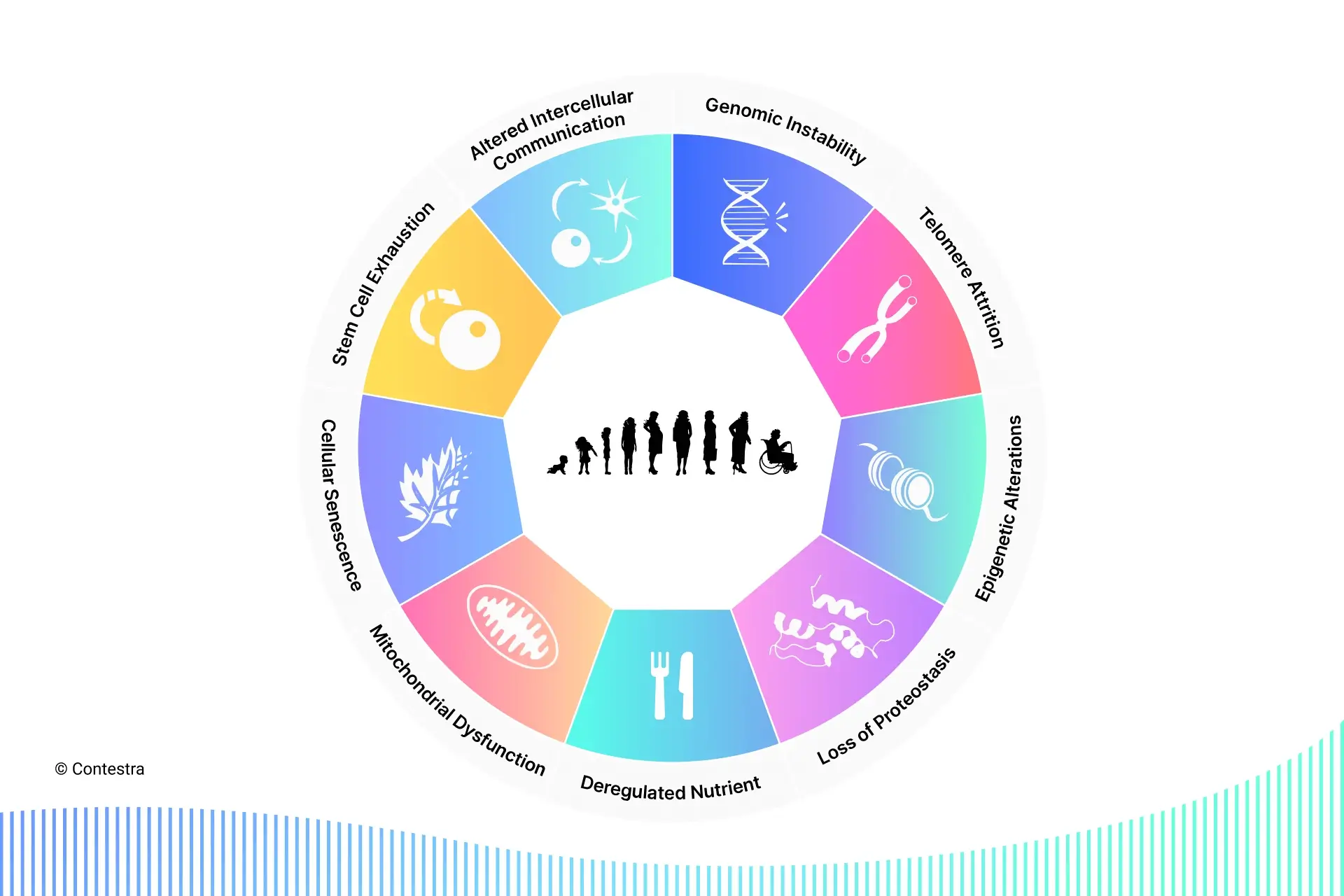
The concept of nine hallmarks of aging, first defined by Carlos Lopez-Otin and colleagues in a 2013 Cell article, is a consensus model that describes the observable damage of aging, which is what ultimately drives age-related pathologies and death. Each hallmark meets the following criteria:
- It should manifest during normal aging.
- Replicating it in the lab should have the same effect as if it happened naturally.
- Its reversal should slow the normal aging process and increase healthy lifespan.
The hallmarks of aging originated from the hallmarks of cancer by Hanahan and Weinberg (six defined in 2000, and ten defined in 2011). The hallmarks of cancer were a paradigm for oncology research, and many scientists agree that hallmarks of aging are a similar paradigm for the gerontology, anti-aging and longevity research.
I’ll introduce each of the nine below.
1. Genomic instability
Genomic instability refers to the increased tendency for DNA mutations and other genetic changes to occur during cell division. Cell divisions occur in our bodies all the time – not only when we cut our finger or scratch our knee and the wound has to heal, but also to replace dead skin cells and keep other body regeneration processes running. DNA damage accumulates in our body over time despite a complex network of DNA repair mechanisms, and in the worst cases leads to cancer formation. This process is at least in part caused by oxidative stress (more on that later), and accelerated by smoke exposure, chemicals and other exogenous agents (such as air pollutants).
2. Telomere attrition
Telomeres are repeating sequences of DNA that sit on the ends of our chromosomes to protect them from the DNA repair machinery. They are often illustrated as the plastic tips on shoelaces (known as aglets) that stop the textile from fraying. Whenever a cell divides, so does each telomere.
However, the DNA-copying enzymes aren’t able to duplicate it all the way to the end, making it shorter with each division. Due to this shortening of the telomere, cells are only able to divide a certain number of times (around 60) before a telomere, this protective tip, doesn’t exist anymore. This is known as the Hayflick limit. Once a cell reaches this limit, it enters a stage of “senescence” (I’ll explain this other hallmark later) – which basically means that the cell becomes a “zombie cell”, excreting inflammatory molecules that damages tissue and promotes disease.
Telomerase is an enzyme that adds base pairs to the ends of our chromosomes (to those shoe aglets) to avoid cells reaching the Hayflick limit. Most cells do not express telomerase, except embryonic stem cells (as with only 60 replications, a baby would never be able to develop in the womb), adult stem cells, male sperm cells, skin cells and immune system cells.
But why don’t we just add telomerase to all the cells? Wouldn’t that make them immortal? Not necessarily. Research has shown that cells that never stop dividing fuel the growth of cancer. It is how cancer tumors form. So, while triggering telomerase might be dangerous, scientists are investigating how to slow down telomere attrition and safely lengthen existing telomeres with social and physical environment changes.
Supplements including omega-3 and vitamin D have been shown to slow telomere attrition. Whereas chronic stress, and childhood adversity has been shown to accelerate telomere attrition.
3. Epigenetic alterations
Epigenome comes from the Greek prefix epi- meaning “outside of” or “around” and describes a record of the chemical changes to the DNA (or genome) of our body. Epigenetics is the study of chemical reactions that influence expression of genes, through activating or deactivating them at specific times and locations.
Epigenome can be illustrated as software, while the DNA is the hardware. The software provides the hardware with the information of how to express genes to suit the cell’s purpose (for example, it is what differentiates a skin cell from a heart cell when they are both made of the same genetic material).
Epigenetics is what causes two genetically identical individuals (identical twins) to be measurably different. Even though their DNA code is the same, the external forces cause the genes to be expressed differently.
An example that directly affects many aging people is epigenetic alterations in the immune system.
Epigenome responds to the biological aging process, but we can also affect it either positively with exercise and positive experiences, such as supportive relationships and opportunities, or negatively with environmental toxins or stressful life events.
The epigenome can be beneficially modified with bioactive nutrition compounds. These include, among others, isothiocyanates in broccoli and other cruciferous vegetables, genistein in soybean, and resveratrol in red grapes and some other purple foods.
4. Loss of proteostasis
Loss of protein homeostasis, or proteostasis, is the loss of our body’s ability to synthesize and degrade proteins. Proteins are complex molecules that regulate almost everything in our body – directly or indirectly. They perform different functions, such as antibody, enzyme, messenger, structural component, or transport and storage. Proteostasis means a balanced state of body in which the protein-producing and degrading machinery works perfectly.
With the loss of proteostasis, mechanisms become less efficient and let the improperly shaped proteins, which would have otherwise been degraded, accumulate and damage cells. This leads to some age-related illnesses such as Alzheimer’s.
5. Deregulated nutrient sensing
Humans evolved in an environment where food supply was uncertain, which made our cells evolve to adjust to the availability of nutrients. Nutrient sensing systems work to maintain metabolic homeostasis (a steady, stable condition).
There are four pathways of nutrient-sensing which regulate metabolism:
- insulin-like growth factor 1 (IGF-1)
- mechanistic target of rapamycin (mTOR)
- sirtuins
- AMP-activated protein kinase (AMPK).
We will be talking about these four pathways in a future article, but for now, it’s enough to understand that they are called “nutrient-sensing” because their activity is influenced by nutrient intake.
Our bodies have DNA repair mechanisms that cannot work while nutrients are abundant. The constant abundance desensitizes the cellular mechanism that sense nutrients.
Obesity and type-2 diabetes are largely diseases of constant exposure to abundant nutrients. Aging also deregulates the nutrient-sensing pathways and causes cells to improperly respond to normal cues that regulate production of energy, cell growth, and other functions.
The scarcity of nutrients that is caused by adequate fasting re-activates repair mechanisms that renew our cells and tissues.
6. Mitochondrial dysfunction
Mitochondria are the cellular power plant. They take the carbohydrates we consume (glucose) and produce energy for our cells (in the form of ATP – adenosine triphosphate). With aging, mitochondria become dysfunctional (they physically swell up and they become fewer) and this reduces our energy levels.
The byproduct of creating ATP are free radicals (reactive oxygen species) which leak into our cells. Even though free radicals are an essential signaling mechanism, they can also damage our cells. As we age, mitochondria produce more free radicals and less energy.
Minimizing oxidative damage (by avoiding oxidative stresses from the environment, like air pollution, cigarettes, radiation, diet high in sugar and processed food, and using antioxidant supplements) can prevent mitochondrial damage and chronic inflammation.
7. Cellular senescence
Our cells can initiate cell death as a protective mechanism against damage. This can take three forms:
- apoptosis — programmed cell death
- autophagy — where faulty cells are broken apart and recycled
- senescence — a form of cell arrest where the cell still functions but can’t divide
Cellular senescence is thought to exist to prevent cancer cells from developing, but it only takes a low level of senescent cells in tissue to cause dysfunction.
The biggest issue of senescent cells is that they start to secrete inflammatory signals (senescence-associated secretory phenotypes or SASPs) and this can cause chronic inflammation if it persists.
An essential part of maintaining a healthy body is tissue renewal, but this carries the risk of cancer. Tumor growth thrives on cell multiplication. Aging well is a compromise between tumor suppression and accumulation of senescent cells.
8. Stem cell exhaustion
Healthy cell renewal is one of the key signs of a healthy life, and stem cells (cells that can develop into any different cell type) are essential to this process. Deficiency of stem cells is directly responsible for common aging issues, such as frailty and weakened immune systems.
Some of the most crucial functions stem cells take on is regulation and replacement of damaged or lost red and white blood cells and solid tissues. As they decline in quality and quantity over time, tissue renewal slows, and diseases start to develop. The more exhausted our body’s stem cell collection, the more opportunity there is for immunosuppression, muscle loss, frailty, and the weakening of bones.
There are ways to stimulate stem cell rejuvenation and renewal – among the most effective are exercise and fasting.
9. Altered intercellular communication
Cells in our body constantly transfer information between each other by secreting signaling molecules either to their neighboring cells or by sending molecular messengers through the bloodstream to affect further cells and tissues. As we age, the signaling systems of chemical messages across the body become more inflammatory, which inhibits the immune system and can potentially cause muscle and bone loss and other harmful effects. We also call this inflammaging. While inflammation is effective as an acute response to disease, when it becomes chronic it is deadly.
Aging is a complex process of interconnected cellular and molecular hallmarks. The hallmarks described in this article serves as a basis for anti-aging research and discussion. In future pieces I’ll describe in detail key hallmarks, starting with cellular senescence.
More Resources: